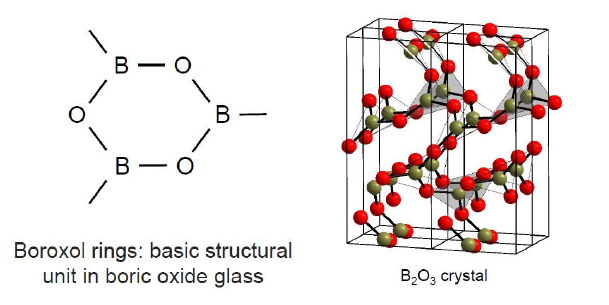
♦Borosilicate glasses mostly contain Quartz silica(SiO2) and Boron Oxide(B2O3) as glass network formers. Borons have corner sharing behavior and make triangle patterns for oxygen bridging.
♦Soda ash(Na2O) or/and Potash(K2O) could be present in batches to modify the network structure. Those alkali ions are responsible for converting the valence of Borons 3 up to 4. However, interaction is not only limited to the valence upgrade of the boron ion; alkaline ions can also bind oxygen and prevent Oxygen-Boron covalent formation.
♦A residual amount of Alumina(Al2O3) could be part of the boronsilicate network. Alumina itself does not have the ability to make a glass network but can vitrify. Although it can improve the chemical resistance (except HF) of glass materials and also have better mechanical properties with localized ductile regions. This attribute differs from the cracking behavior of glass. Rather than brittle cracking, alumina allows bending to some extent before crack propagation begins. This is a result of a new mechanical property called "viscous creep".
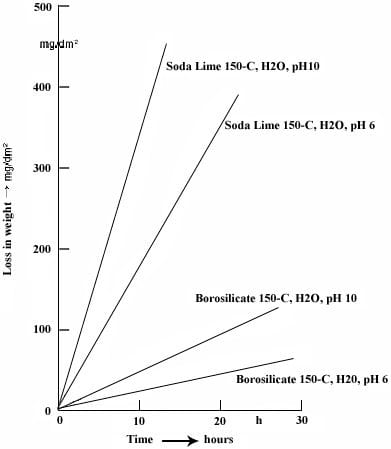
♦Another important properties of borosilicate glasses are thermal shock resistance and low thermal expansion. More heat resistant even at alkaline environment compared to soda-lime glasses. Boronsilicate glasses are mostly used in industrial equipments, exterior lighting, laboratory and kitchen glasswares.