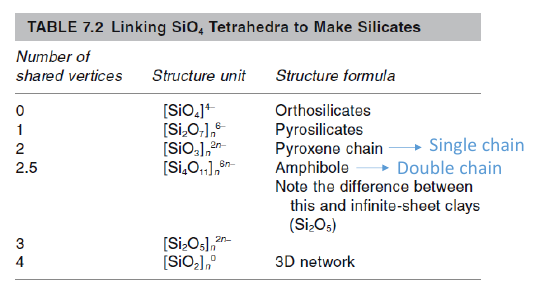
♦Coordination number of silicates is 4. Since its surrounded with four oxygen atom, it has -4 charge in total[SiO4]4- that would be used for make bond with other ions. Anions(Si) could being shared in corner, face and edge positions. Silicas can be found in different various formations like chains, rings and sheets and different cations(Na,Ca,Al,Fe,Mg,K) also play role to determine their structure. Olivine is free form, mica is sheet, bentonite is T-ring, amphibole is doublechain and quartz,feldspar,zeolite are framework.
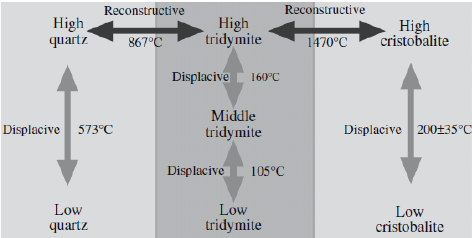
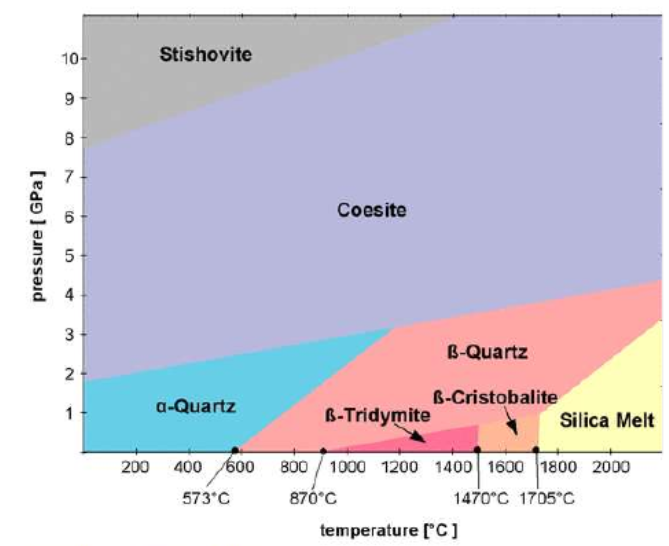
♦ They have framework structure, high strength at elevated temperatures, gas permability at firing stage with no deformation. a-Quartz, b-Quartz, b-Tridymite, b-Cristobalite are common polymorphs. In some cases, polymorphic transformations could be displacive.
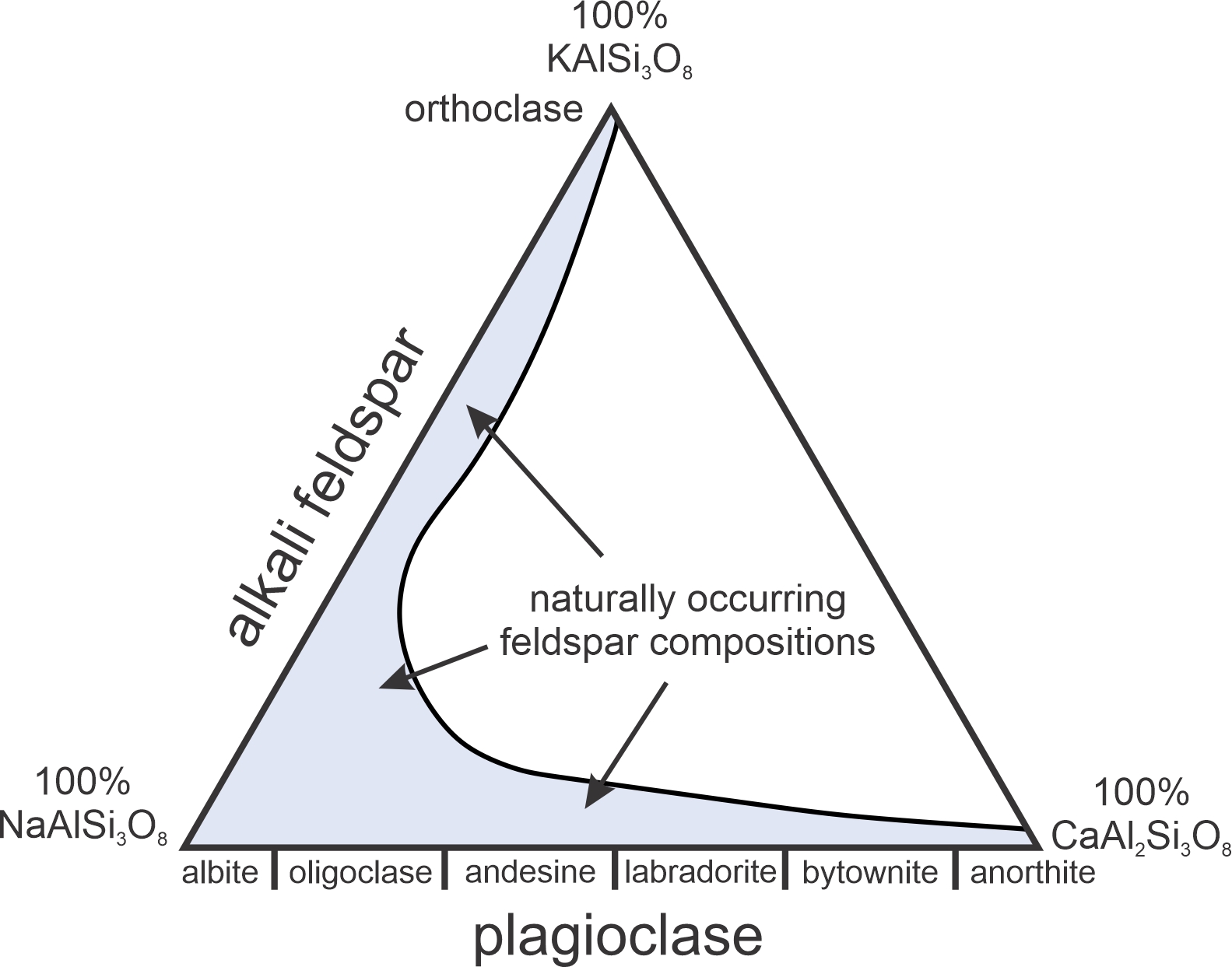
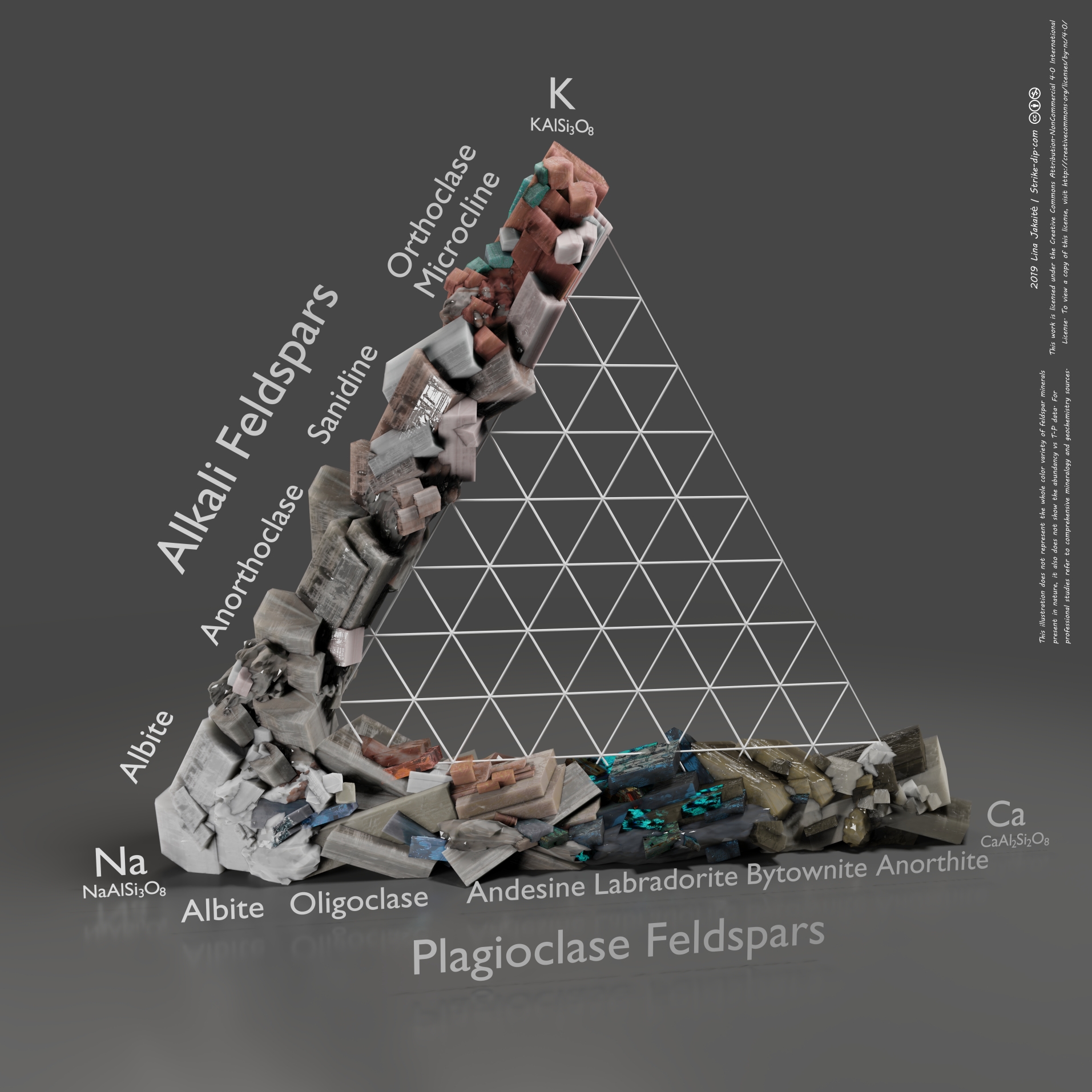
♦Having same coordination number with silicates, like Alumina can be replaced with Si4+. Alumina needs a large cation with +1 or +2 charge(like Ca) since its surrounded with 8 or more oxygen atoms. Alumina has superior properties especially against mechanical scratches and thermal shock resistance. Can be vitrifying even at lower temperatures and with alkali ions does not dissolve in water. e.g Orthoclase K(AlSi3)O8, Albite Na(AlSi3)O8, Anorthite Ca(Al2Si2)O8.
♦Feldspar is in alumina silicate class and has framework structure and 70% of its mineral source is currently being used in glass industry. Two main variants are the alkali (K-Na) feldspars and the plagioclase (Ca-Na) feldspars. Additionally, Ca+2 and Al+3 are also can be present with continuous different compositions in Na+ and Si+4.
♦
♦